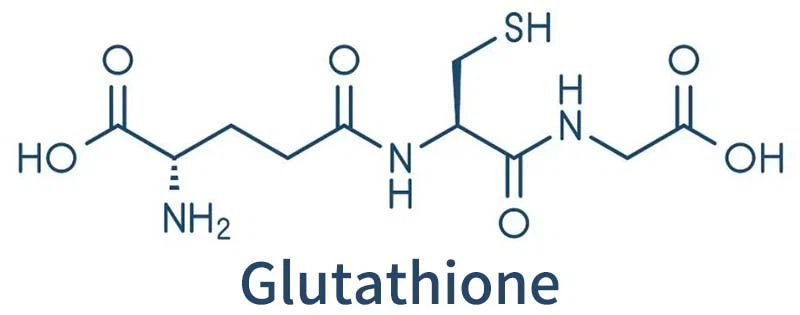
Glutathione is a safe antioxidant composed of glutamic acid, cysteine and glycine that is widely used in pharmaceuticals, foods and cosmetics.
Glutathione is mainly produced industrially by yeast, and the yield is increased by genetic modification and culture technology optimization. However, more glutathione accumulates in yeast cell production, which is not conducive to high yield.
The team of Professor Shingo Kobayashi from Kobe University in Japan published a paper entitled “Engineering Escherichia coli for efficient glutathione” in the journal Metabolic Engineering In this study, the method of omics analysis and metabolic engineering was used to modify Escherichia coli to produce glutathione efficiently by thiosulfate, which enriched the fermentation process of Escherichia coli for glutathione.

【 Result 】
1, Glutathione production in Escherichia coli
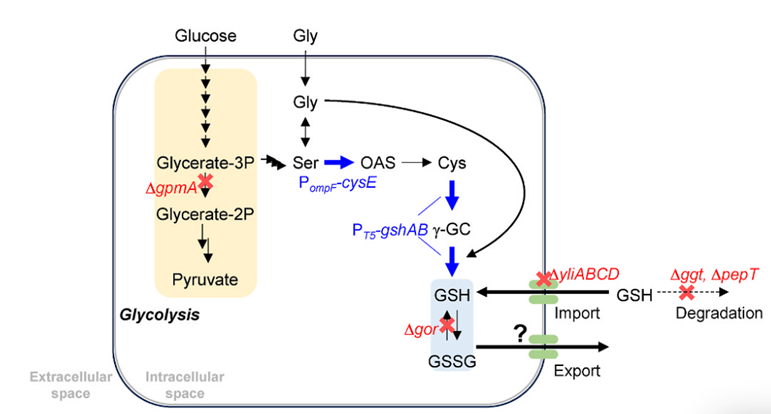
Firstly, gshA (encoding glutamate ligase) and gshB (encoding glutathione synthase) were heterogeneously expressed in Escherichia coli, indicating that Escherichia coli can produce and secrete glutathione without adding cysteine using glucose as carbon source.
The analysis of the total amount of glutathione and GSSG in the fermentation culture showed that, Glutathione accumulates in the cell mostly in reduced form (GSH), while glutathione in supernatant oxidized to GSSG.
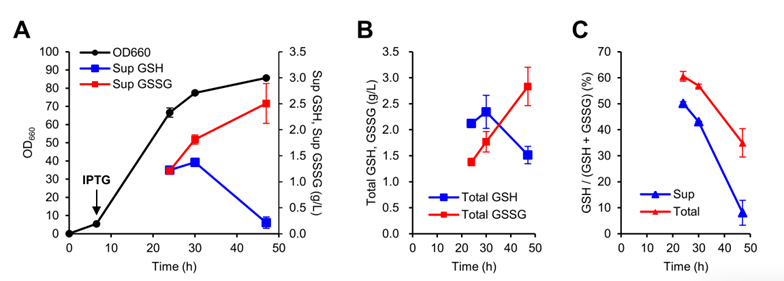
2, Genetic engineering improves glutathione production
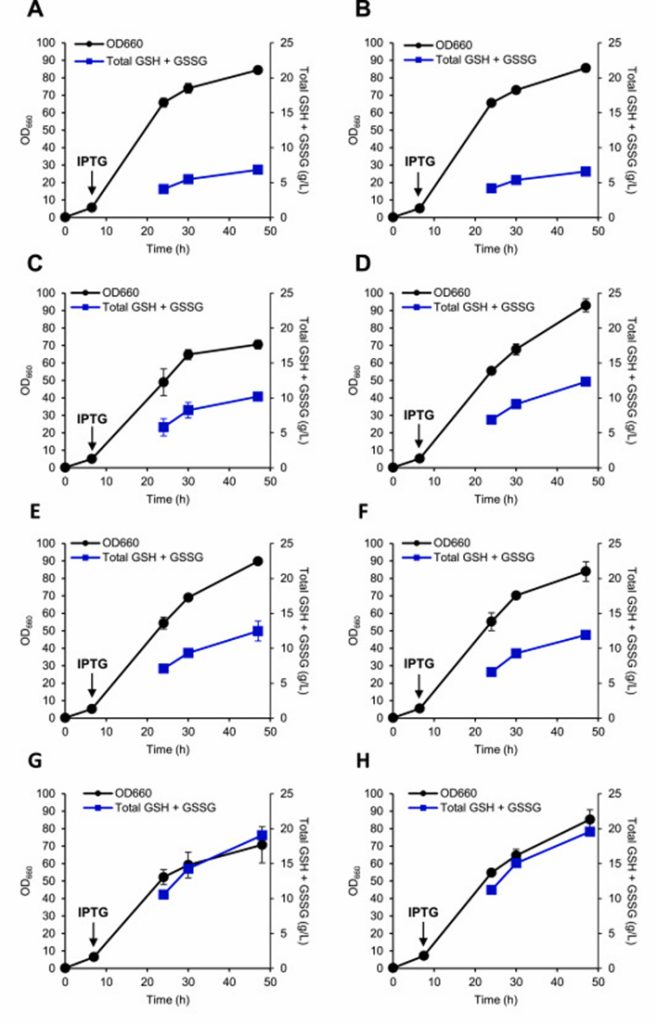
ggt (gamma glutamyltransferase), pepT (tripeptide peptidase) and gor (glutathione reductase) knocked out to inhibit the degradation of glutathione.
As a result, GSH production increased, but GSH production comparable, suggesting that ggt and pepT may not participate in the degradation of intracellular GSH.
gor has a negative regulating effect on the increase of total GSH and GSSG, but can not increase the GSH ratio.
YliABCD is an ABC transporter involved in the reuptake of GSH secreted into the periplasm.
After knocking out yliABCD, the uptake of GSH secreted outside the cell inhibited and its efflux promoted, resulting in increased glutathione production.
Further enhancement of cysteine synthesis by expression of cysE results in a significant increase in GSH production, indicating that the conversion of serine to O-ACe-tyrosine is a key rate-limiting step.
Finally, the glycolysis pathway partially blocked, the carbon flux of 3PG (glycerol 3-phosphate) to serine increased, and the gpmA encoding gene of glycerol phosphomutase knocked out, and the yield slightly increased.
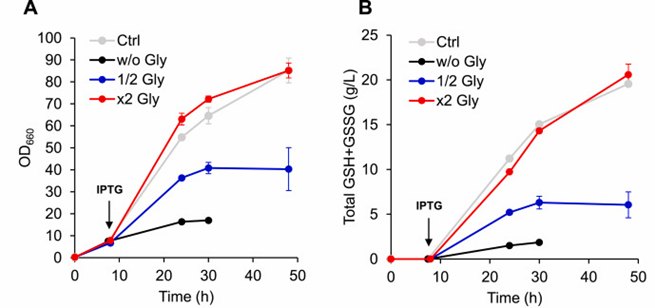
3, Optimization of glycine supplement rate
Because both cysteine and glycine biosynthesized by serine, the researchers focused on the relationship between glycine supplementation and glutathione production.
It found that the growth of the strain severely inhibited and the yield of GSH + GSSG was very low with or without the addition of glycine, indicating that glycine supplementation played an important role in the production of glutathione in Escherichia coli.
The growth of the strain accelerated and the content of GSH + GSSG not significantly increased after the supplementation rate of glycine further increased.
4, Metabolic flux analysis MFA
Although the rate of glycine supplementation had a key effect on glutathione production, excessive glycine supplementation only accelerated strain growth and did not significantly improve GSH production, suggesting that a portion of glycine not used for glutathione production and may metabolized as a carbon source.
We continuously added 75 mM, 150 mM, or 300 mM of unlabeled glycine to fermentation and studied glycine metabolism in cells using [U-13C] D-glucose and unlabeled glycine.
The results showed that in all cases, the growth of strains significantly affected by the addition of glycine, the consumption of glycine increased linearly, the added glycine completely consumed, and the excess glycine used as a carbon source.
The concentration of 13C in protein amino acids analyzed by GC/MS. Results On the surface, the concentration of 13C in glycine and serine gradually decreased, and the supplemented glycine converted to serine.
In contrast, the 13C enrichment of aspartate and threonine remained around 100%, indicating that glycine not converted to threonine, and thus, supplemented glycine used for limited biological processes, such as protein production and glutathione production.
Further analysis of the source ratio of serine and metabolic flux distribution showed that under all conditions, serine mainly derived from glycerol 3-phosphate, and o-acetylserine OAS or OAS derivatives not fully converted to glutathione, and there may metabolic accumulation or spillover.
![Figure 4 Metabolic flux analysis (MFA) was performed on strains cultured in a 200 ml bioreactor with [U-13C] D-glucose and three different concentrations of unlabeled glycine.](https://biowoo.com/wp-content/uploads/2024/08/download-4.jpg)
5, Metabolomics analysis
The results of metabolic flux analysis showed that a part of carbon lost during the metabolism of serine to GSH during glutathione production.
In order to determine the rate-limiting reaction, 150 mM of glycine continuously added during fermentation, and the extracellular and extracellular metabolites in the glutathione biosynthesis pathway investigated by LC-MS/MS analysis.
Results Serine, OAS, n-acetyl-L-serine (NAS), cysteine and gamma-GC temporarily accumulated in and out of the cell, and OAS levels peaked at 30 hours.
In contrast, intracellular NAS concentrations are several times higher than OAS and mainly detected in the supernatant, which is mainly related to the physiological instability of OAS, which spontaneously isomerizes into NAS.
The researchers determined that the conversion of OAS to cysteine is a key rate-limiting step in glutathione biosynthesis.
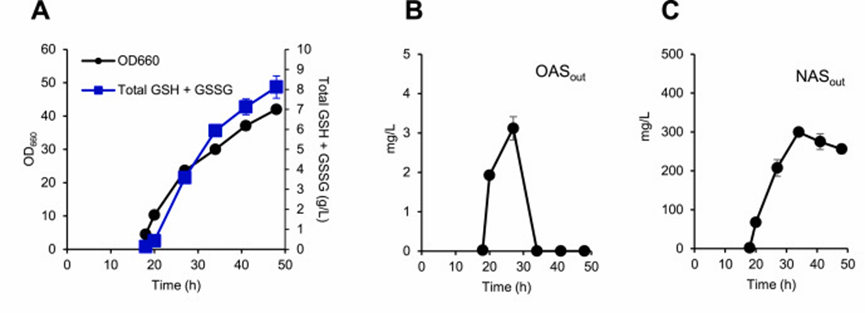
6, Metabolomics analysis of KG06 cultured in 200 mL bioreactor
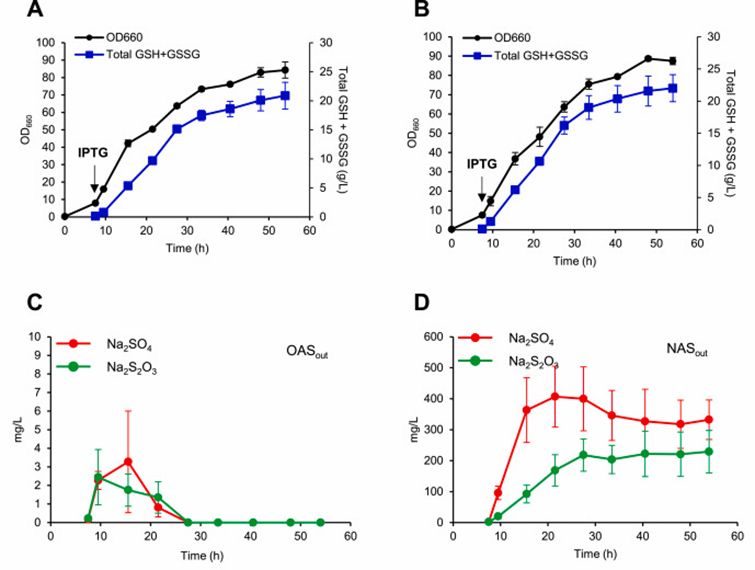
The results of MFA and metabolomics analysis showed that the conversion of OAS to cysteine was a rate-limiting reaction. Therefore, cysK and cysM coding genes overexpressed in KG06 strain.
Overexpression of cysK would have a negative effect on glutathione production, and overexpression of cysM would lead to unstable cell growth. When glycerin and acetate were the only carbon sources, the titer and yield were relatively low.
Thiosulfate considered a better source of sulfur because it requires lower NADPH consumption to reduced to sulfide, so the researchers tested whether it could used as a sulfur source to accelerate cysteine production.
The results showed that the use of Na2S2O3 increased glutathione production when cultured with Na2S2O3 or Na2SO4 supplemented with equal amounts of sulfur.
Metabolomics analysis showed that both intracellular and extracellular OAS concentrations not affected by sulfur sources.
In contrast, the intracellular and extracellular NAS concentrations were significantly lower with Na2S2O3, confirming that Na2S2O3 accelerates the conversion of OAS to cysteine.
【 Summary 】
In this paper, we first established a fermentation process of Escherichia coli, which can produce GSH without adding cysteine, and further inhibited glutathione degradation, inhibited GSSG reduction and enhanced cysteine synthesis by gene modification to change metabolic flux.
Combined with metabolic flux analysis and metabolomics analysis, the rate-limiting steps of glutathione biosynthesis determined. The use of thiosulfate as a sulfur source in fermentation alleviated this limitation, and finally achieved a GSH yield of 22.0 g/L.